Breakthrough extraction-free PCR testing for infectious diseass @ANYWHERE™
THE PROBLEM
13M global deaths annually from infectious diseases.
People lack access to affordable, accurate, rapid diagnostic testing.
PCR is the gold standard, but reliance on slow, costly extraction keeps it confined to centralized labs, ERs, and minute clinics. Transformative Biotech removes this barrier with patented extraction-free technologies that enable direct testing from clinical samples, lowering cost and accelerating turnaround so PCR can reach point-of-care settings and help reduce millions of preventable deaths through earlier diagnosis.
WHY PCR REMAINS STUCK IN THE LAB
Despite advances in portability, existing PCR devices still rely on traditional extraction workflows, instrument-dependent processes, and controlled lab-like environments. Some portable systems have made it into clinics, but they have not eliminated the core barriers: purification steps, inhibitory sample media, cost, and workflow complexity.
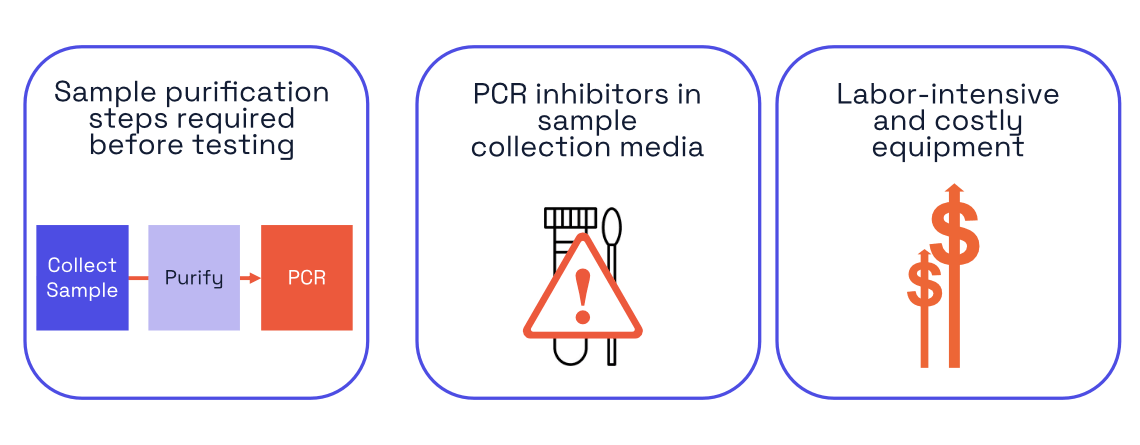
As a result, point-of-care PCR systems that rely on extraction remain costly and difficult to scale, and most PCR testing is still confined to centralized or near-centralized labs.
OUR TECHNOLOGY
Patented Breakthrough Extraction-Free PCR
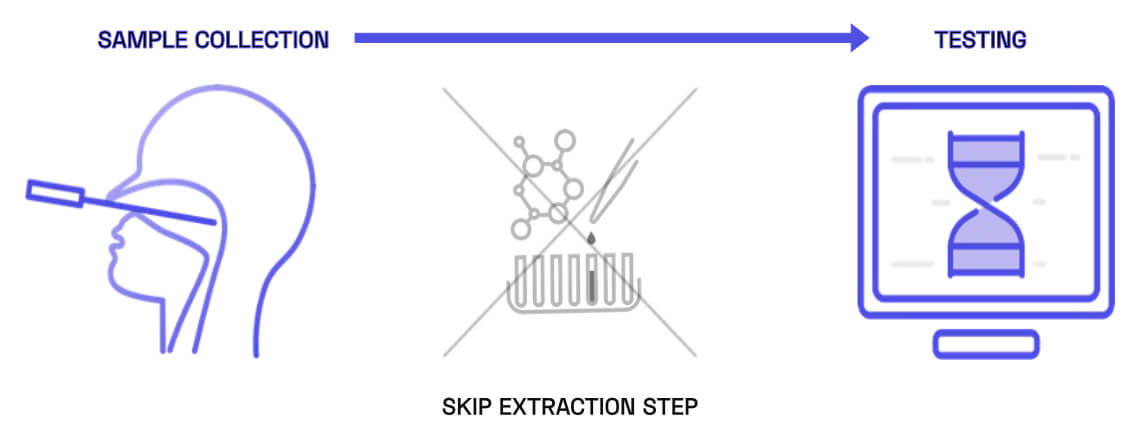
Our patented direct-to-PCR technologies eliminate the key barriers that limit traditional PCR workflows. By removing the need for sample purification, overcoming inhibitory media, and enabling high-performance amplification directly after collection, we enable PCR testing that is faster, simpler, and broadly accessible. Our chemistry can be integrated into existing PCR workflows or future point-of-care systems, removing the extraction bottleneck that has limited where PCR can be used. Our goal is to democratize PCR so accurate diagnostics can be delivered @ANYWHERE™.
Compared to conventional PCR:
7x faster turnaround times
7x lower cost average across test types
Highly accurate results
COMPETITIVE ADVANTAGE
Our patented chemistry eliminates nucleic‑acid extraction, enabling high‑performance PCR directly from raw swab and saliva samples and delivering accurate, rapid, scalable testing across platforms.
We are advancing toward commercialization and strategic partnerships in research, clinical testing, and next-generation point-of-care diagnostics. This will position us as a foundational enabler for next-generation molecular diagnostics.
Competitive Comparison
Our Chemistry
✓ Extraction-free
✓ Non-inhibitory formulation
✓ Works with swabs and saliva
✓ Compatible with standard PCR systems
✓ Patent-backed (1 issued, 5 pending)
Traditional PCR / Other Media
✗ Requires extraction
✗ Contains PCR inhibitors
✗ Limited saliva performance
✗ Lab-dependent workflows
✗ Fragmented IP landscape
THE EVIDENCE
Issued U.S. Patent 12,344,889
Extraction-Free Pathogen Testing Methods
Issued July 1, 2025
- Core extraction-free PCR chemistry and methods supporting direct PCR amplification from swabs, saliva, and other sample types without nucleic acid extraction.
- Five additional patents pending, covering platform extensions and device integration.
Peer-Reviewed Publication
Independent research and external validation support the performance of our extraction-free platform:
- Demonstrated clinical equivalence to traditional extraction-based PCR in high-volume settings.
- Proof-of-concept studies beyond COVID-19, including respiratory pathogens and STIs (CT/NG).
Real-World Clinical Evidence
- More than 590,000 patient samples processed in a CLIA-certified, high-volume diagnostic laboratory (Summit Biolabs).
- $14M in commercial revenue generated through extraction-free workflows (Summit Biolabs).
- Consistent real-world performance and scalability demonstrated in routine clinical testing.
Ongoing Technology Development
- Advancing handheld, smartphone-enabled PCR concept for @ANYWHERE™ use.
- Co-development discussions with instrument and assay developers.
- Focused on commercialization and strategic partnership pathways to enable direct-to-PCR technologies with the power to transform molecular testing for infectious diseases and cancer.
TEAM
Kevin Kraus (CEO) brings senior leadership experience in diagnostics and molecular testing from roles at Roche, Genentech, and multiple startups. Shelle Pourmanafzadehardabili (VP Operations) previously led operational scale-up, clinical validation, and quality systems at Summit Biolabs.
Board of Directors: Bob Blomquist (Board Chair, Co-Inventor) brings diagnostics and pharma leadership experience, as well as direct IP strategy. Russ Hullet (Co-Founder and former Co-CEO) has investment experience and was the lead investor at Summit Biolabs. Dr. Barbara Handelin (former Co-CEO) provides industry and scientific insight as a pioneer in molecular genetics.
Transformative Biotech grew out of work at the University of Colorado Anschutz, where extraction-free PCR chemistry was first developed to streamline oncology assays. During the pandemic, Summit BioLabs applied it at scale for COVID testing, revealing its broader value. Three team members formed a new company around the technology and recruited Barbara, a long-time collaborator of Bob. Kevin joined in Q3 2025 with a passion for bringing innovation to patients, shaped by his early years at the start-up Ventana Medical Systems. Together, the team is motivated to remove workflow barriers in PCR and expand access to molecular diagnostics.
Advisors & Collaborators
We collaborate with leading experts and academics across diagnostics, engineering, and infectious diseases.






